
|
A short Curriculum Vitae of the authors |

![]()
Chewing Gum as a Drug Delivery
System
Biradar S S1 Bhagavti
S T1 Hukkeri V I 1
Rao K P 2 Gadad A P1
![]()
Introduction: Man has a habit of chewing the chewing gum
since ancient times. Today it is one of the most popular dosage form, used for
delivering the many active components.
The first medical chewing gum was introduced in market in
1928 consisting of aspirin an analgesic drug. However, chewing gum did
not gain acceptance as a reliable drug delivery system until 1978, when
nicotine chewing gum became available in 1980,
Most of the chewing gum
were used for smoking cessation (containing the nicotine) and also used for
oral and dental hygiene (consisting of fluoride and carbamide etc).
Chewing gum can be used as drug delivery for many active
components. With the inclusion of medical chewing gum in the European
Pharmacopoeia in 1998, have further contributed to the acceptance of this
method of drug delivery.
Today, medical
chewing gum meets the same high-quality standards as tablets and can be
formulated to obtain different release profiles of active substances, thus
enabling distinct patient group targeting.
![]()
What are the ingredients in chewing gum?
Active substances and additives sweeteners like
sorbitol mannitol and suitable fruity flavours and nonsticky Gum Base The gum base is the insoluble part left in
the mouth while chewing and it is a polymer. The gum base is made of
resins from trees, latexs or the milky juices from plants, and manmade
polymers. If the gum base is chicle from the sapodilla tree, this product
is being harvested in
|
|
Cuts on the sapodilla tree let the sap run into a collection bucket. |
Chicle is boiled over an open
fire in the rainforest to evaporate some of the excess water. Once it is
thick and taffy-looking, it is packed into wooded forms to make blocks.
These blocks are shipped to some American chewing gum manufacturers.
Chicle is a rubbery latex or polyterpene. Polyterpenes are composed of
thousands of C5H8 isoprene subunits.
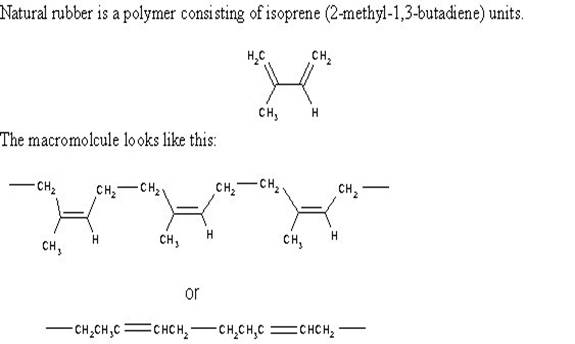
Today, the gum base could also be made
from styrene butadiene, poly (vinyl acetate) or polyethylene. The sugar
is for sweetening the product. The corn syrup keeps the gum fresh and
flexible. Softeners or fillers such as vegetable oils help to blend the
ingredients and retain moisture. Sugar free gum has sorbitol, mannitol,
aspartame or saccharin instead of sugar. The gum base determines the basic
characteristics of the product example texture whether it is soft? Does it
crumble? Does it stick to the teeth?



![]()
Why
we use chewing gum as a drug delivery
system?
There are many reasons for selecting the chewing as a
drug delivery system, the following are the some reasons highlighted.
1) Easy for administration without water promotes higher
patient compliance.
2) Children and for patients who find swallowing tablets
difficult are obvious.
3) Local effect
4) Systemic Effect
5) Fast onset of action
6) Less side effects
7) Less risk of overdosing
8) Effective on Dry mouth
![]()
High acceptance in
children
Many children find it difficult to swallow tablets. To overcome
this problem, liquid formulations have been developed, however, administering
liquid formulations may be difficult and circumstantial as well. A chewing gum
formulation is an obvious alternative. In a chewing gum formulation, it is most
often possible to disguise the bitter/bad taste of the active substance, making
it a pleasant experience for the child. However, it is important that the child
chews the chewing gum for the prescribed period of time. Compared to a liquid
formulation, chewing gum also provides easier Storage as there is no
risk of microbial contamination.
![]()
Local therapy
Prevention and
cure of oral diseases are obvious targets for chewing gum formulations. Chewing
gum can release an active substance at a controlled. Sugar-free chewing gum is
known to be beneficial to dental health. It has been shown that use of
sugar-free chewing gum after meals re-elevates plaque pH 1
Indications for fluoride chewing gum are prevention of dental
carries in Children in fluoride-deficient areas, in adults with a high incidence
of carries, and in patients with xerostomia. The carries-preventive effect of
fluoride chewing gum has been compared with the effect of placebo chewing gum
in oral infections caused by bacteria or fungi are often seen, especially in
patients with impaired immune system. Chlorhexidine Chewing gum can be used for
treatment of gingivitis, peridontitis and other oral and pharyngeal infections4 it can also be used for inhibition of plaque growth and
has shown Successful treatment of
minor pains, headaches, pains of cold, muscular aches, etc. requires rapid
absorption of therapeutic doses of the active substance. Chewing gum as a drug
delivery system could be beneficial in minor pain treatment, when buccal
absorption results in fast onset of action and reduces the risk of
gastrointestinal side effects. The bioavailability of acetylsalicylic acid in a
chewing gum formulation relative to an unbuffered tablet formulation has been
determined8.
A chewing gum formulation may also be useful in the treatment of
acute, strong pain. Bioavailability of methadione from a chewing gum
formulation has been compared to a tablet formulation. There was no
significant difference in the bioavailability of the two formulations9.
![]()
Patient
compliance
As no water is required, taking medication in chewing gum is
very convenient and therefore suitable for acute treatment. The medication may
be taken without regard to time and place, thus promoting compliance.
Chewing gum does not draw attention to the medication, it is discrete
and does not Stigmatize the patient.
Today, there is a trend towards higher patient involvement in
drug administration and handling. Chewing gum is in line with this trend as it
allows easy self-administration and does not prevent patients from living an
active life. Further Clinical trials involving patients with oral candidosis
have shown that miconazole chewing gum is at least as efficient as miconazole
oral gel in the treatment of fungal infections in the mouth6,7.
![]()
Fewer side effects
Active substances absorbed buccally bypass the hepatic first
pass metabolism, which may result in a higher bioavailability of the active
substance. Thus, the equivalent efficacy may be obtained with a lower dosage, and
consequently less side effects are expected. Further, a lower dosage may
reduce the risks of interactions with other active substances. The controlled
release rate also reduces the risk of side effects, as high plasma peak
concentrations are avoided.
![]()
Effect on dry mouth
Dry mouth is a side effect of many types of medication (e.g.
antidepressants), and it is also part of the symptomatology of several diseases
(e.g. Sjögren’s syndrome). It is well known that chewing gum stimulates
salivary secretion1, and a chewing gum formulation therefore partly alleviates this
condition. Furthermore, as dry mouth increases the incidence of dental caries,
chewing gum may also be beneficial to dental health. It has been shown that
long-term activation of the salivary glands by chewing gum several It has been
shown that long-term activation of the salivary glands by chewing gum several
times per day for two months enhanced resting salivary flow, especially in
individuals with low salivary flow1.
![]()
Less risk of overdosing
Chewing is required to release the active substance from chewing
gum. If the chewing gum is swallowed accidentally, only limited amounts of the
active substance will be released over a relatively long period of time, thus
reducing the risk of high plasma peak concentrations and overdosing.
![]()
Therapeutic uses:
The Chewing gum
can be used for therapeutic purposes by incorporating the various medicaments
especially antiasthamatic and Nonsteroidal anti-inflammatory and antiobsedrugs.
![]()
Evaluation
of chewing gum: The absorption of active substances through the buccal mucosa
can be examined by both in vitro and in vivo methods. The most common in
vitro method involves an Using chamber, in which excised buccal mucosa
from either humans or animals is placed as a barrier between two chambers.The
transport of active substances across the mucosa is measured by withdrawal of
samples from each chamber. Buccal mucosa from domestic pigs is recommended,
mainly because of the morphological similarity in mucosa from the human and
porcine oral cavities.
Likewise, a human TR146 cell culture model has proven a good in
vitro model for investigating permeability, permeability mechanisms, effects of
chemical enhancers, and toxic effects18.
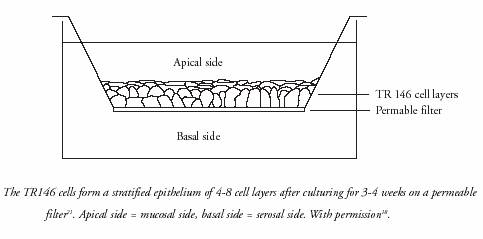
The machines
are driven by air and are set to a specific number and frequency of chews
inside a water bath at 37 degrees Celsius, similar the temperature of saliva in
a person's mouth. Once the gum is "chewed" the fluid is tested to see
how much of the drug has been released. The results are used to evaluate
effectiveness and to develop new gum products25

Buccal absorption of active substances can also be tested
by various in vivo methods. Beckett and Triggs introduced a mouth wash procedure
in 1967, in which a buffered solution of the active substance is swirled in the
oral cavity for a known period of time23.
Subsequently, the solution is expelled and the oral
cavity is rinsed with buffer. The difference between the amount of active
substance contained in the original solution and the amount recovered is
assumed to be the amount of active substance absorbed from the oral cavity.
Since the introduction of this method, it has been improved by various modifications, however, the main limitation
lies in the fact that the method cannot
account for storage of active substances in the mucosa.
Another in vivo method involves a perfusion
chamber, which is adhered to the
buccal mucosa of the test person. The absorbed amount of active substance perfused through the chamber is
calculated as the decrease in active
substance24.
![]()
Conclusion
Though chewing gum as a drug delivery system has currently
gained wide acceptance only within smoking cessation and oral healthcare, vast
interest in this mode of drug delivery for a wide variety of other indications
exists and continues to grow. Clinical trials
have confirmed the advantages to be gained by exploiting the effects of chewing
gum, per se, the convenience of the delivery and the
possibilities of buccal absorption and local effect. Furthermore, one
trial has indicated that chewing gum is possibly a safer drug delivery system
for active substances that are susceptible to abuse. As chewing gum as a drug
delivery system is to be expanded into additional therapeutic areas, it is
important that the delivery form is acceptable to the end-users. Clinical
trials and market research have proven this to be the case. In the coming
years, new formulations will enter the market and chewing gum will become a much
more common drug delivery system. _
Date:17.2.2005.
Corresponding Author
1) S
Lecturer Lecturer
Department of pharmaceutics Department of pharmaceutics
Vidyanagar,
Vidyanagar,
HUBLI-580031. HUBLI-580031.
Email:: biradarappu4u@rediffmail.com
![]()
References
1. Imfeld, T. (1999) Crit. Rev. Oral Biol. Med. 10, 405-419.
2. Lamb, W.J. et al. (1993) Caries Res. 27, 111-116.
3. Sjögren, K. et al. (2002) Caries Res., in press.
4. Smith, A.J. et al. (1996) J.
Clin. Periodontol. 23,
19-23.
5. Simons, D. et al. (1999) British Dent. J. 187, 612-615.
6. Rindum, J.L. et al. (1993) Scand. J. Dent. Res. 101, 386-390.
7. Rindum, J.L. et al., in preparation.
8. Woodford, D.W., Lesko, L.J. (1981) J. Pharm. Sci. 70, 1341-1343.
9. Christrup, L.L. et al. (1990) Acta Pharm. Nord. 2, 83-88.
10. Rassing, M.R. (1994) Adv. Drug
Del. Rev. 13,
89-121.
11. Jensen, E.J. et al. (1991) Psychopharmacol. 104, 470-474.
12. Odusola, F. (1991) The
13. Olsson, H. et al. (1991) Acta Odontol. Scand. 49, 273-279.
14. Rhodus, N.L., Schuh, M.J. (1991) Oral Surg. Oral Med. Oral Pathol. 72, 545-549.
15. Avidan, B. et al. (2001) Aliment Pharmacol. Ther. 15, 151-155.
16. Schönfeld, J.v. et al. (1997) Digestion 58, 111-114.
17.
Drug Delivery,
Marcel Dekker, Inc.,
18. Nielsen, H.M. (2000) Ph.D. thesis, HCØ Tryk,
19. Nielsen, H.M. (2002) In: Lehr C.-M.
(Ed.) Cell Culture Models of
Biological Barriers: In vitro Test Systems for Drug Absorption
and Delivery,
Harwood Academic Publishers,
20. Collins, L.M.C., Dawes, C. (1987) J.
Dent. Res. 66, 1300-1302.
21. Jacobsen, J. et al. (1995) Int. J. Pharm. 125, 165-184.
22. Nielsen, H.M., Rassing, M.R. (2002) Eur. J. Pharm. Sci.,
submitted.
23. Beckett, A.H., Triggs, E.J. (1967) J. Pharm. Pharmacol. 19, 31S-41S.
24. Rathbone, M.J. et al. (1996) In: Rathbone, M.J. (Ed.) Oral Mucosal
Drug Delivery,
Marcel Dekker, Inc.,
25. Dr. Gord McKay September 2004www.innovationplace.com
![]()
![]()
© Copyright-VIPAPHARM. All rights reserved

![]()
